 Clinicians on the frontlines are faced with big questions about testing for themselves, patients and their own families. It is hard to keep up with the changing information and availability, so we have prepared this article compiled from the Stanford Medicine Anesthesia Informatics and Media Lab to help understand the two tests that are available for COVID-19 testing.
Clinicians on the frontlines are faced with big questions about testing for themselves, patients and their own families. It is hard to keep up with the changing information and availability, so we have prepared this article compiled from the Stanford Medicine Anesthesia Informatics and Media Lab to help understand the two tests that are available for COVID-19 testing.
There are two categories of tests that are available: molecular and serological. They can be differentiated by testing objectives. One tests if people are infected with the virus and the other tests if they have developed antibodies to fight the virus.
A molecular test for COVID-19 detects viral genetic material by using reverse transcription polymerase chain reaction, or RT-PCR. The genetic material from the nose mucous or saliva sample is copied and then compared to the geneticsequence of the virus you’re trying to detect.
The serological test uses a blood sample to detectimmunoglobulin M (IgM) to show recent exposure to sars-cov-2 and the presence ofimmunoglobulin G (IgG) antibodies that show later-stage infection, recovery and possible immunity.
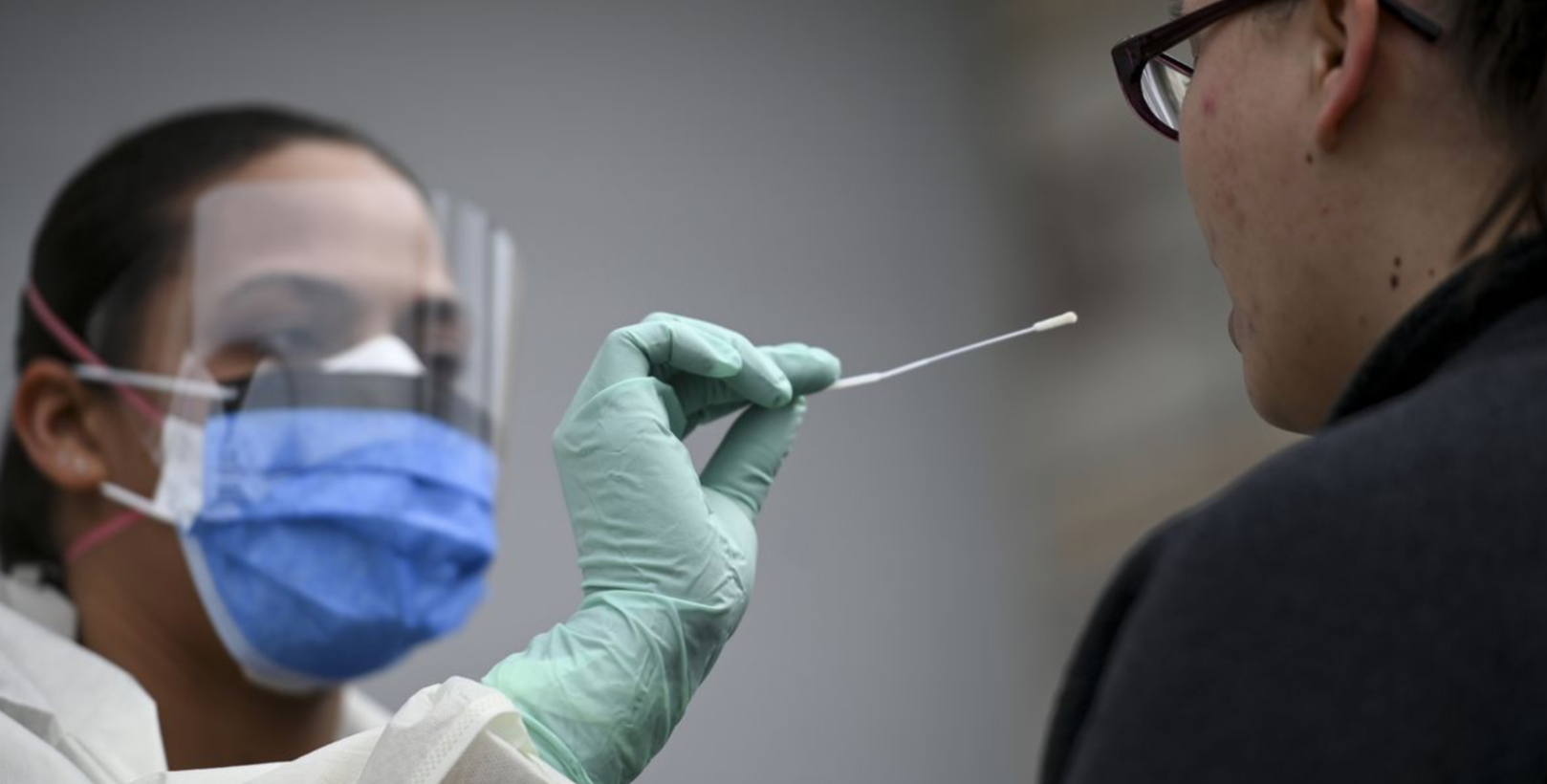
Molecular Testing
Molecular tests are done by inserting a 6-inch long swab into the back of one nasal passage and rotating the swab for 15 seconds and then repeated for the other nostril. The swab is sent to a lab for testing. These tests can be done in an office, a drive by clinic, and soon at home with results sent to a lab. A saliva test with similar accuracy to the swab test and less pain was developed recently. The FDA authorized the first at home molecular testing this April. The swabs with these kits only need to go as far as the nostril. Results of testing can be ready in minutes but many labs are taking about a week to return results. The accuracy differences between the types of molecular swabsneed further investigation with larger, high quality studies.
Dr. Winslow Murdoch shares how to do home molecular testing.
Serological Testing
Serological tests rely on detecting antibodies in a blood sample, usually obtained through a finger prick that can be processed at the point of care, but samples can also be derived from plasma or serum. When you’re exposed to the virus, your body develops antibodies, and the time it takes to develop them varies from person to person. IgM is the largest immunoglobulin, and appears first after initial exposure to an antigen. IgG immunoglobulin G antibodies are plentiful, occur 5-10 days or after infection and show later-stage infection, recovery and possible immunity.
Serological tests use a technique called enzyme-linked immunological assay (ELISA), which detects the presence of these antibodies to the virus. Exposure to similar infections may produce antibodies but not confer immunity against the current virus.
To summarize, one test finds when you have the virus (which informs treatment) and the other show when you have immunity to the virus. Children can be tested too and this guide shares hints for how to prepare your child.
The challenge is that none of these tests are independently evaluated by the FDA standards, but only by internal manufacturer testing augmented by sporadic testing from independent scientists for claims of accuracy.
There is no vaccine, interventions are not established and the testing we have is unpredictable. As diagnostic and treatment options evolve policy will shift according to available evidence. In this age of uncertainty, it is important to withhold early judgement and remain flexible, curious and kind.

